Transcription-dependent HIV latency
Zhao group at Central China Normal University
A computational study of Tat-CDK9-Cyclin binding dynamics and its implication in transcription-dependent HIV latency
HIV is a virus that attacks the T cells. HIV may either actively replicate or become latent within host cells for years. Since HIV uses its own protein Tat to hijack the host CDK9-Cyclin complex for transcription, Tat is implicated in transcription-dependent HIV latency. To quantify the impact of Tat binding, we propose a computational framework to probe the dynamics of CDK9-Cyclin interface and the ATP pocket reorganization upon binding by different Tat mutants [1]. Specifically, we focus on mutations at three Tat residues P10, W11, and N12 that are known to interact directly with CDK9 based on the crystal structure of Tat-CDK9-Cyclin complex. Our molecular dynamics simulation shows that the CDK9-Cyclin interface becomes slightly weaker for P10S and W11R mutants but tighter for K12N mutant. Furthermore, the side-chain orientation of residue K48 in the ATP pocket of CDK9 is similar to the inactive state in P10S and W11R simulations, but similar to the active state in K12N simulations. This framework may hence help gain a better understanding on the role of Tat in the transcription-dependent HIV latency establishment.
1. The interface angle anlysis for Native, P10S, W11R, and K12N:
To analyze the dynamical motions of the CDK9-Cyclin interface, we calculated the interface angle for native, P10S, W11R, and K12N during the simulations. The interface angle is defined by the following atoms: N (LYS68, CDK9), OD1 (ASN179, CDK9), and NZ (LYS159, Cyclin). These are angle files of five 200 ns (a total of 1000 ns) MD simulations for each state.
Download
2. The interface correlation analysis for Native, P10S, W11R, and K12N:
We performed the dynamical network correlation analysis to probe the interface interactions qualitatively. The interface residues of CDK9-Cyclin were identified by GETAREA.33 A residue is identified as the interface residue if it is solvent-exposed in either CDK9 or Cyclin alone but not in the CDK9-Cyclin complex. We performed dynamical network correlation analysis for native, P10S, W11R, and K12N mutant simulations, respectively. These are correlation files of five 200 ns (a total of 1000 ns) MD simulations for each state.
The interface residues:
(1) CDK9: PRO11, PHE12, GLU57, ILE61, LEU64, LYS68, GLN71, ARG86, LYS96.
(2) Cyclin: ARG11, GLU96, GLN131, ILE135, SER138, GLN142, GLY145, PHE146.
Download
3. The conformational changes of ATP-binding residues:
To investigate the conformational changes of ATP-binding residues upon Tat mutations, we performed side chain RMSD calculations during the last 30 ns MD simulations to detect the conformational changes of the ATP-binding residues for Native, P10S, W11R, and K12N. These are RMSD files of five 200 ns (a total of 1000 ns) MD simulations for each state.
Download
4. Tat sequence variation analysis:
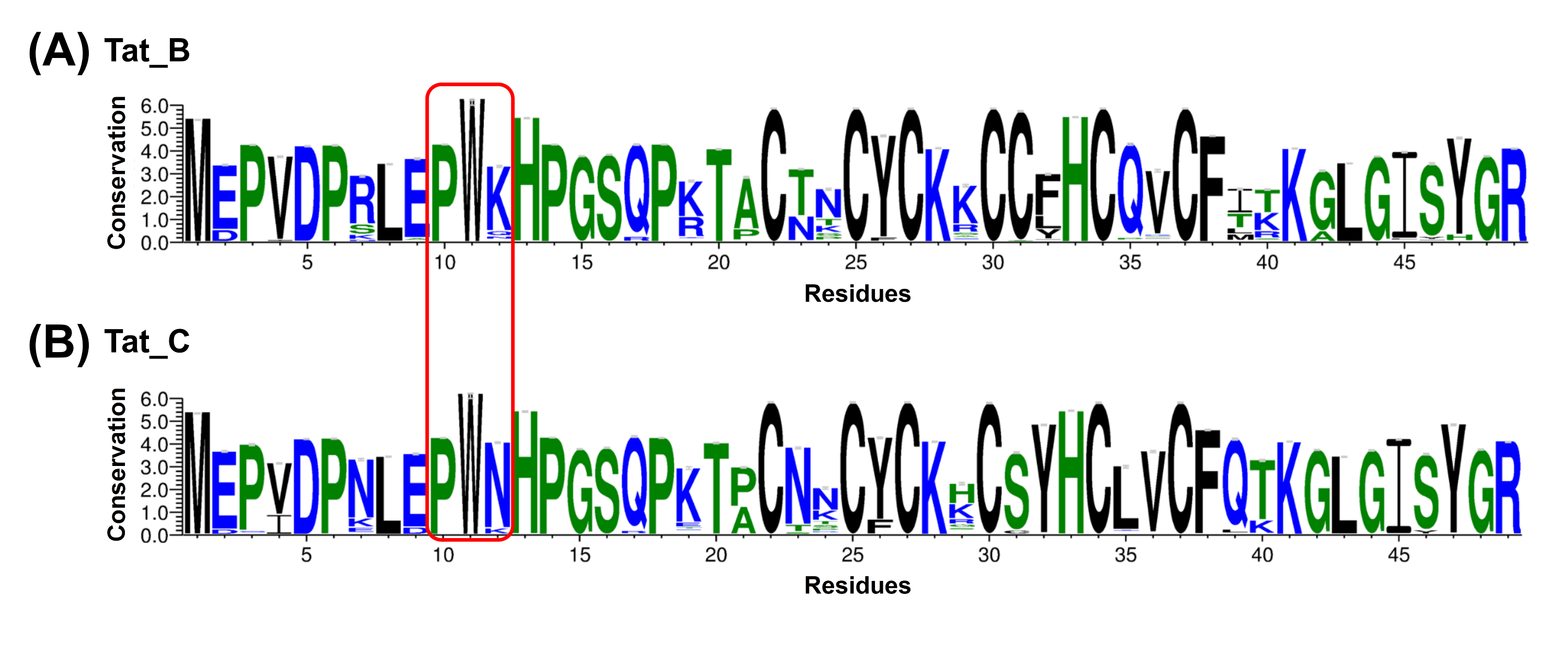
We analyzed the Tat sequence variations in HIV-1 subtypes B and C. The overall height of the WebLogo symbols indicates the relative sequence variation of each amino acid at a particular position. The P10 and W11 in Tat are relatively conserved. The HIV-1 infection process may need conserved P10 and W11. The P10S and W11R mutations would cause HIV latency which agrees with our computational results. The K12 in Tat is variable. Besides, K12 is conserved in HIV subtype B and N12 is conserved in HIV subtype C. The Tat K12N mutant binding to the p-TEFb made CDK9-CyclinT interface stronger and presumably made p-TEFb more active that leads to stronger kinase activity on CTD of RNA Pol II and thus less latency for Clade C. These computational results are consistent with the general latency of Clade B vs Clade C [2,3].
FIG. Sequence variations of Tat of HIV-1 subtypes B (A) and C (B). The overall height of the WebLogo symbols indicate the relative sequence variation of each amino acid at the particular position. The P10 position and W11 position are relatively conserved while K12 position is more variable.
Download
References
1. H. Wang, L. Song, T. Zhou, C. Zeng, Y. Jia, Y, Zhao. A computational study of Tat-CDK9-Cyclin binding dynamics and its implication in transcription-dependent HIV latency. Phys. Chem. Chem. Phys. DOI: https://doi.org/10.1039/D0CP03662E.
2. D. Kamori, T. Ueno, HIV-1 Tat and viral latency: what we can learn from naturally occurring sequence variations. Front. Microbiol 8, 80 (2017).
3. V. R. Rao, U. Neogi, J. S. Talboom, L. Padilla, M. Rahman, C. Fritz-French, S. Gonzalez-Ramirez, A. Verma, C. Wood, R. M. Ruprecht, Clade C HIV-1 isolates circulating in Southern Africa exhibit a greater frequency of dicysteine motif-containing Tat variants than those in Southeast Asia and cause increased neurovirulence. Retrovirology 10, 61 (2013).
Copyright 2019, Lab of Biophysics